I'm referring to the curve of temperature to percentage of decarboxylation over time. In other words, a chart of time/temp to achieve full decarboxylation.
My understanding is that "full" conversion is not possible, but there is a maximum possible
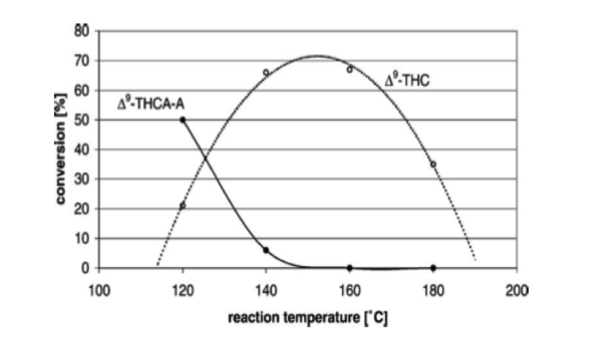
Ahh, you mean something like this

and then this
 https://cornerstonecollective.com/getting-familiar-with-thca/
https://cornerstonecollective.com/getting-familiar-with-thca/As seen, the issue is not so simple as “increasing temperature increases THCA to THC conversion”. After a certain point, THC observed begins to disappear. This is because at certain temperatures, some of the THC molecules begin to oxidize to form cannabinol (CBN), a non-psychoactive cannabinoid. However, due to the nature of the chemical reactions taking place, THC begins oxidizing to CBN before all of the THCA contained in a sample can be converted to THC. The two reactions are occurring simultaneously. This is important to note for two reasons:
- It is therefore impossible to successfully convert to THC, all THCA from a given sample. In this research group’s experiment, the highest conversion rate was revealed to be around 70%. Although some cannabis dispensaries add THC and THCA amounts together as the total amount of THC listed within a sample, this is simultaneously accurate and misleading. Consumers armed with knowledge of the behavior of THCA as a precursor will be able to more easily estimate the effect of the THCA contained within a given sample.
- This explains why creating cannabis edibles can be so sensitive; although heat is needed for decarboxylation, heat over 150 degrees Celsius (~300 degrees Fahrenheit) will begin to also degrade the THC. To attain the maximum potency of medicine with the least residual THCA or CBN, bakers must aim for temperatures around 140-160 Celsius.

